The introduction of trifluoromethyl groups into drug molecules can effectively prolong the duration of action and enhance the metabolic stability of the drug molecules. Therefore, compounds containing trifluoromethyl groups have been widely used in the pharmaceutical field. For example, Prozac, a drug for mental depression, Cilazapro, a drug for arthritis and Genovent, a drug for type II diabetes, Sorafenib tosylate, an anti-cancer drug, and Fluoxetine, an anti-depressant, all contain trifluoromethanes and have the structural formulae shown in Figure 1.
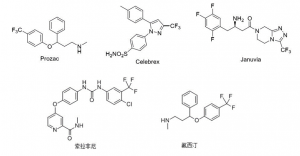
Figure 1. Trifluoromethyl compounds in the pharmaceutical field
Trifluoromethyl compounds have a footprint in many therapeutic areas, for example, aliphatic compounds that act as anaesthetics, as well as phenothiazine tranquillisers and antiemetics, benzothiazide diuretics, and some compounds that have not been clinically evaluated, such as antihistamines, antiasthmatic, antimalarial and anti-staphylococcal.
Trifluoromethyl compounds can be obtained by trifluoromethylation reactions. Currently, commonly used trifluoromethylation reagents can be TMSCF3, CF3SO2Na, Togni’s reagent, Umemoto’s reagent, BBDFA’s reagent, CF3I, etc. as trifluoromethyl sources. Using CF3I trifluoroiodomethane as the trifluoromethyl source, Tetsu Yamakawa’s research team has achieved a single-step process for the industrial production of 5-trifluoromethyluracil from uracil and CF3I using a reagent consisting of FeSO4, H2O2 and dimethyl sulfoxide (DMSO). Examples of the reactions are as follows.
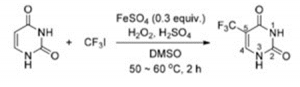
5-Trifluoromethyluracil (TFU) is a key intermediate in the synthesis of the anticancer drug trefoil thymidine. Triflumethymidine is a spectroscopic antiviral drug that inhibits herpes simplex virus and herpes zoster virus, as well as HSV-I,HSV-II, CMV, varicella virus and certain adenoviruses [1].
A reagent consisting of CF3I and FeSO4, H2O2 and dimethyl sulfoxide (DMSO) can trifluoromethylate various bases (uracil), providing an industrial trifluoromethylation process [2]. This method uses uracil and trifluoroiodomethane (CF3I) as the main raw materials, and the target product is obtained by one-step trifluoromethylation. The reaction route is short, but suitable process conditions need to be found before industrialisation can be achieved. Trifluoromethyl-modified uracil is a key intermediate in some anticancer drugs. The reaction routes are as follows.
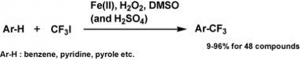
WuXi AppTec New Drug Development Co., Ltd. invented a patent involving a practical synthesis method for 5-trifluoromethyluracil. It mainly solves the technical problems of the existing synthesis methods of 5-trifluoromethyluracil, which have many or expensive synthesis steps and high cost due to excessive use of raw materials. Using uracil as the raw material, 5-trifluoromethyluracil can be obtained in high yield by heating the reaction with trifluoroiodomethane, hydrogen peroxide and aqueous iron sulphate in a fluoroboric acid environment [3].
2,4-Dichloro-5-trifluoromethylpyrimidine is widely used as a useful pharmaceutical intermediate in the preparation of various fluoropyrimidine-containing drugs [4]. The precursor is 5-trifluoromethylpyrimidine, which is subjected to chlorination to give the product.
Trifluoroiodomethane is used as a trifluoromethylation reagent, or it can be used directly as a gas without making a solution. Gas mixtures can be made by diluting with argon, ammonia, air, helium, oxygen, etc. to make a molar ratio of 1 to 100% of trifluoroiodomethane for use. The reaction is carried out in a closed system or in an open system where the trifluoroiodomethane or the gas mixture is introduced into the reaction solution by bubbling [5].
Iron compounds, trifluoroiodomethane, sulfoxides and peroxides, and also trifluoromethylation reagents containing acids, can be used to trifluoromethylate various organic substrates, in particular compounds with an enamine site, furans, thiophenes and benzenes can be effectively trifluoromethylated. As compounds with an enamine site, such as N-vinyl lactams, uracil, pseudouracil, thymine, cytosine, adenine, guanine, hypoxanthine, xanthine, pyrazoles, indoles, pyrroles, triazoles, anilines, pyridines, pyrimidines, pyrazines, etc., a variety of important pharmaceutical intermediates such as purines, pyrimidines, pyridines and pyrazoles containing trifluoromethyl groups are obtained.
α,β-unsaturated structures appear in many important pharmaceutical synthesis intermediates, and the trifluoromethylated products of α,β-unsaturated aldehydes and their derivatives can be hydrolysed under specific conditions to produce active trifluoromethyl ketone structures [6]. 2009 MacMillan’s research team at Princeton University used CF3I as the trifluoromethylated structure catalyzed by an organic small molecule catalyst and a photocatalyst to The reaction route for the introduction of CF3- into the α-position of an asymmetric aldehyde catalyzed by an organosmall molecule and photocatalyst is shown in Figure 2.
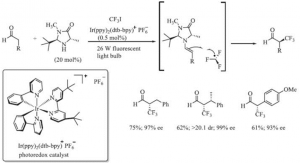
Figure 2. Introduction of CF3- to the α-position of an asymmetric aldehyde using CF3I as the trifluoromethyl source
Trifluoromethyl ketones are a particularly important class of compounds among the many trifluoromethyl-containing molecules, with strong biological activity as inhibitors of a variety of enzymes; they are also important intermediates for the synthesis of other trifluoromethyl derivatives.
Ref.
[1] A method and process for the preparation of 5-trifluoromethyluracil.
[2] Trifluoromethylation of various aromatic compounds by CF3I in the presence of Fe(II) compound, H2O2 and dimethylsulfoxide. Tatsuhito Kino, Yu Nagase, Yuhki Ohtsuka, Kyoko Yamamoto, Daisuke Uraguchi, Kenji Tokuhisa and Tetsu Yamakawa. A Journal of Fluorine Chemistry 131 (2010) 98 -105.
[3] Synthesis method of 5-trifluoromethyluracil, Patent CN200910057588.3, Tianjin WuXi AppTec New Drug Development Co.
[4] A method for the synthesis of 2,4-dichloro-5-trifluoromethylpyrimidine, a fluoropyrimidine-containing pharmaceutical intermediate, Patent,CN108503592 A
[5] Reaction Reagent for Trifluoromethylation, Patent, WO2008/056677, Sagami Central Research Institute of Chemistry, Toso Fluorine Technology Co.
[6] Visible light-catalyzed trifluoromethylation of α,β-unsaturated aldehydes and their derivatives.